Imago via ZUMA
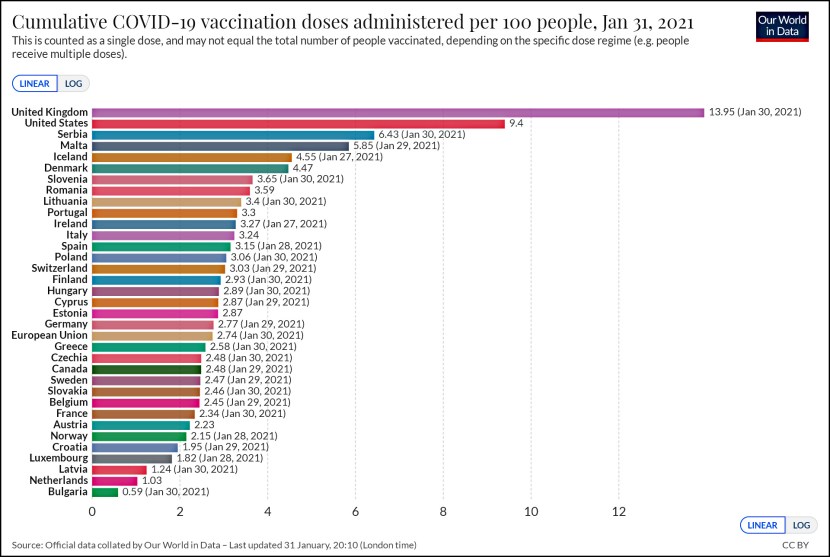
For some reason this doesn’t seem to be common knowledge, so it’s worth mentioning that the FDA’s vaccine advisory committee will be meeting again this Thursday to discuss emergency approval of a COVID-19 vaccine. This time it’s the Moderna vaccine, and there’s every reason to think they will recommend approval and the FDA will grant it. The United States purchased 100 million doses last summer and another 100 million doses a few days ago. Since the Moderna vaccine requires two shots, this is enough for 100 million people.
Between the Pfizer vaccine and the Moderna vaccine we will have enough to innoculate 150 million people, which will probably take us through mid-2021 at the rate we can reasonably expect the vaccines to be distributed.
This is good news, and there’s more: the Moderna vaccine appears to be slightly more effective than the Pfizer vaccine, and it can be stored in ordinary refrigerators for up to a month. This should make distribution much easier in more places than the Pfizer vaccine.
Between these two vaccines, as well as others coming down the pike, the US should be well supplied with enough vaccine to provide an innoculation to everyone who wants one over the next year or so. Ditto for other countries. It’s the beginning of the end.