A few years before Super Size Me hit theaters in 2004, Dr. Paresh Dandona, a diabetes specialist in Buffalo, New York, set out to measure the body’s response to McDonald’s—specifically breakfast. Over several mornings, he fed nine normal-weight volunteers an egg sandwich with cheese and ham, a sausage muffin sandwich, and two hash brown patties.
Dandona is a professor at the State University of New York-Buffalo who also heads the Diabetes-Endocrinology Center of Western New York, and what he observed has informed his research ever since. Levels of a C-reactive protein, an indicator of systemic inflammation, shot up “within literally minutes.” “I was shocked,” he recalls, that “a simple McDonald’s meal that seems harmless enough”—the sort of high-fat, high-carbohydrate meal that 1 in 4 Americans eats regularly—would have such a dramatic effect. And it lasted for hours.
Inflammation comes in many forms. The swelling of a sprained ankle indicates repairing torn muscle and tendon. The redness and pain around an infected cut signifies the body’s repulsion of microbes. The fever, aches, and pains that accompany the flu represent a body-wide seek-and-destroy mission directed against an invading virus. They’re all essential to survival, the body’s response to a perceived threat or injury. But inflammation can also cause collateral damage, especially when the response is overwhelming—like in septic shock—or when it goes on too long.
Chronic, low-grade inflammation has long been recognized as a feature of metabolic syndrome, a cluster of dysfunctions that tends to precede full-blown diabetes and that also increases the risk of heart disease, stroke, certain cancers, and even dementia—the top killers of the developed world. The syndrome includes a combination of elevated blood sugar and high blood pressure, low “good” cholesterol, and an abdominal cavity filled with fat, often indicated by a “beer belly.” But recently, doctors have begun to question whether chronic inflammation is more than just a symptom of metabolic syndrome: Could it, in fact, be a major cause?
For Dandona, who’s given to waxing grandiloquent about the joys of a beer on the porch in his native Delhi, or the superb ice wines from the Buffalo region, the results presented a quandary. Food was a great pleasure in life. Why would Nature be so cruel, he wondered, and punish us just for eating?
Over the next decade he tested the effects of various foods on the immune system. A fast-food breakfast inflamed, he found, but a high-fiber breakfast with lots of fruit did not. A breakthrough came in 2007 when he discovered that while sugar water, a stand-in for soda, caused inflammation, orange juice—even though it contains plenty of sugar—didn’t.
The Florida Department of Citrus, a state agency, was so excited it underwrote a subsequent study, and had fresh-squeezed orange juice flown in for it. This time, along with their two-sandwich, two-hash-brown, 910-calorie breakfast, one-third of his volunteers—10 in total—quaffed a glass of fresh OJ. The non-juice drinkers, half of whom drank sugar water, and the other half plain water, had the expected response—inflammation and elevated blood sugar. But the OJ drinkers had neither elevated blood sugar nor inflammation. The juice seemed to shield their metabolism. “It just switched off the whole damn thing,” Dandona says. Other scientists have since confirmed that OJ has a strong anti-inflammatory effect.
Orange juice is rich in antioxidants like vitamin C, beneficial flavonoids, and small amounts of fiber, all of which may be directly anti-inflammatory. But what caught Dandona’s attention was another substance. Those subjects who ate just the McDonald’s breakfast had increased blood levels of a molecule called endotoxin. This molecule comes from the outer walls of certain bacteria. If endotoxin levels rise, our immune system perceives a threat and responds with inflammation.
Where had the endotoxin come from? One possibility was the food itself. But there was another possibility. We all carry a few pounds’ worth of microbes in our gut, a complex ecosystem collectively called the microbiota. The endotoxin, Dandona suspected, originated in this native colony of microbes. Somehow, a greasy meal full of refined carbohydrates ushered it from the gut, where it was always present but didn’t necessarily cause harm, into the bloodstream, where it did. But orange juice stopped that translocation cold.
Dandona’s ongoing experiments—and others like it—could upend much of we thought we knew about the causes of obesity, or just that extra pesky 10 pounds of flab. If what some scientists now suspect about the interplay of food and intestinal microbes pans out, it could revolutionize the $66 billion weight loss industry—and help control the soaring $2.7 trillion we spend on health care yearly. “What matters is not how much you eat,” Dandona says, “but what you eat.”
EVER SINCE THE DUTCH DRAPER Antonie van Leeuwenhoek first scrutinized his own plaque with a homemade microscope more than three centuries ago and discovered “little living animalcules, very prettily a-moving,” we’ve known that we’re covered in microbes. But as new and cheaper methods for studying these microbes have become available recently, their importance to our health has grown increasingly evident. Scientists now suspect that our microbial communities contribute to a number of diseases, from allergic disorders like asthma and hay fever, to inflammatory conditions like Crohn’s disease, to cancer, heart disease, and obesity.
As newborns, we encounter our first microbes as we pass through the birth canal. Until that moment, we are 100 percent human. Thereafter, we are, numerically speaking, 10 percent human, and 90 percent microbe. Our microbiome contains at least 150 times more genes, collectively, than our human genome. Think of it as a hulking instruction manual compared to a single page to-do list.
As we mature, we pick up more microbes from breast milk, food, water, animals, soil, and other people. Sometime in childhood, the bustling community of between 500 and 1,000 species stabilizes. Some species are native only to humans, and may have been passed down within the family like heirlooms. Others are generalists—maybe they’ve hopped aboard from pets, livestock, and other animal sources.
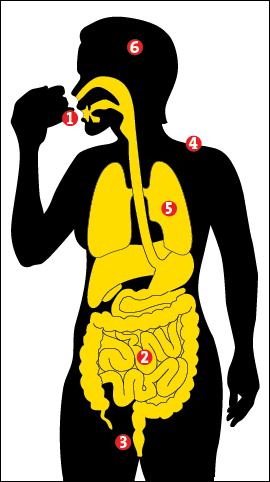
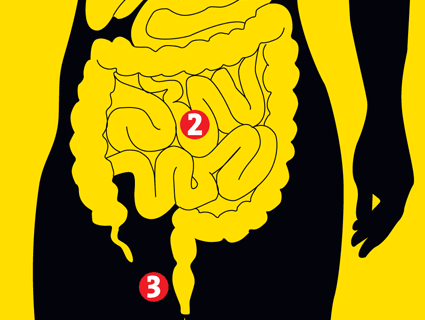
Most of our microbes inhabit the colon, the final loop of intestine, where they help us break down fibers, harvest calories, and protect us from micro-marauders. But they also do much, much more. Animals raised without microbes essentially lack a functioning immune system. Entire repertoires of white blood cells remain dormant; their intestines don’t develop the proper creases and crypts; their hearts are shrunken; genes in the brain that should be in the “off” position remain stuck “on.” Without their microbes, animals aren’t really “normal.”
What do we do for our microbes in return? Some scientists argue that mammals are really just mobile digestion chambers for bacteria. After all, your stool is roughly half living bacteria by weight. Every day, food goes in one end and microbes come out the other. The human gut is roughly 26 feet in length. Hammered flat, it would have a surface area of a tennis court. Seventy percent of our immune activity occurs there. The gut has its own nervous system; it contains as many neurons as the spinal cord. About 95 percent of the body’s serotonin, a neurotransmitter usually discussed in the context of depression, is produced in the gut.
So the gut isn’t just where we absorb nutrients. It’s also an immune hub and a second brain. And it’s crawling with microbes. They don’t often cross the walls of the intestines into the blood stream, but they nevertheless change how the immune, endocrine, and nervous systems all work on the other side of the intestine wall.
Science isn’t always consistent about what, exactly, goes wrong with our microbes in disease situations. But a recurrent theme is that loss of diversity correlates with the emergence of illness. Children in the developing world have many more types of microbes than kids in Europe or North America, and yet generally develop allergies and asthma at lower rates than those in industrialized nations. In the developed world, children raised in microbially rich environments—with pets, on farms, or attending day care—have a lower risk of allergic disease than kids raised in more sterile environments.

Those who study human microbial communities fret that they are undergoing an extinction crisis similar to the one afflicting the biosphere at large—and that modern medicine may be partly to blame. Some studies find that babies born by C-section, deprived of their mother’s vaginal microbes at birth, have a higher risk of celiac disease, Type 1 diabetes, and obesity. Early-life use of antibiotics—which tear through our microbial ecosystems like a forest fire—has also been linked to allergic disease, inflammatory bowel disease, and obesity.
Which brings us to the question more and more scientists are asking: If our microbiota plays a role in keeping us healthy, then how about attacking disease by treating the microbiota? After all, our community of microbes is quite plastic. New members can arrive and take up residence. Old members can get flushed out. Member ratios can shift. The human genome, meanwhile, is comparatively stiff and unresponsive. So the microbiota represents a huge potential leverage point in our quest to treat, and prevent, chronic disease. In particular, the “forgotten organ,” as some call the microbiota, may hold the key to addressing our single greatest health threat: obesity.
PARESH DANDONA LEFT INDIA in 1966 for a Rhodes Scholarship at Oxford University. He became “the first colored guy,” he says, to head his unit at the University of London hospital. His bearing—heels together, back stiff, and an orator’s care with words delivered in a deep, sonorous voice—recalls a bygone era. He moved to Buffalo in 1991.
During those decades, the number of Americans considered obese nearly tripled. One-third of Americans are now considered overweight, and another third obese. Worldwide, one-fourth of humanity is too heavy, according to the World Health Organization. In 2011, the United Nations announced that for the first time ever, chronic diseases, most of which are linked to obesity, killed more people than infectious diseases. In the United States, obesity accounts for 20 percent of health care costs, according to Cornell University economists.
And the problems aren’t limited to the obese themselves: Children born to obese mothers have hardened arteries at birth, a risk factor for cardiovascular disease. They have a greater risk of asthma. Some studies suggest they’re more likely to suffer from attention deficit disorders and autism.
Why are we increasingly prone to obesity? The long-dominant explanation is simply that too little exercise and too many calories equals too much stored fat. The solution: more exercise and a lot more willpower. But there’s a problem with this theory: In the developed world, most of us consume more calories than we really need, but we don’t gain weight proportionally.
A pound of body fat contains roughly 3,500 calories. If you run a daily surplus of just 500 calories—the amount in a bagel with a generous serving of cream cheese—you should, judging by the strict calorie-in-must-equal-calorie-out model, gain a pound of fat per week. Most of us do run a surplus in that range, or even higher, but we either gain weight much more slowly, or don’t gain weight at all.
Some corpulent people, meanwhile, have metabolisms that work fine. Their insulin and blood sugar levels are within normal range. Their livers are healthy, not marbled with fat. And some thin people have metabolic syndrome, often signaled by a beer gut. They suffer from fatty liver, insulin resistance, elevated blood sugar, high blood pressure, and low-grade, systemic inflammation. From a public health perspective, these symptoms are where the real problem lies—not necessarily how well we fit into our jeans.
Here’s the traditional understanding of metabolic syndrome: You ate too much refined food sopped in grease. Calories flooded your body. Usually, a hormone called insulin would help your cells absorb these calories for use. But the sheer overabundance of energy in this case overwhelms your cells. They stop responding to insulin. To compensate, your pancreas begins cranking out more insulin. When the pancreas finally collapses from exhaustion, you have diabetes. In addition, you develop resistance to another hormone called leptin, which signals satiety, or fullness. So you tend to overeat. Meanwhile, fat cells, which have become bloated and stressed as they try to store the excess calories, begin emitting a danger signal—low-grade inflammation.
But new research suggest another scenario: Inflammation might not be a symptom, it could be a cause. According to this theory, it is the immune activation caused by lousy food that prompts insulin and leptin resistance. Sugar builds up in your blood. Insulin increases. Your liver and pancreas strain to keep up. All because the loudly blaring danger signal—the inflammation—hampers your cells’ ability to respond to hormonal signals. Maybe the most dramatic evidence in support of this idea comes from experiments where scientists quash inflammation in animals. If you simply increase the number of white blood cells that alleviate inflammation—called regulatory T-cells—in obese mice with metabolic syndrome, the whole syndrome fades away. Deal with the inflammation, it seems, and you halt the dysfunction.
Now, on the face of it, it seems odd that a little inflammation should have such a great impact on energy regulation. But consider: This is about apportioning a limited resource exactly where it’s needed, when it’s needed. When not under threat, the body uses energy for housekeeping and maintenance—and, if you’re lucky, procreation, an optimistic, future-oriented activity. But when a threat arrives—a measles virus, say—you reprioritize. All that hormone-regulated activity declines to a bare minimum. Your body institutes a version of World War II rationing: troops (white blood cells) and resources (calories) are redirected toward the threat. Nonessential tasks, including the production of testosterone, shut down. Forget tomorrow. The priority is to preserve the self today.
This, some think, is the evolutionary reason for insulin resistance. Cells in the body stop absorbing sugar because the fuel is required—requisitioned, really—by armies of white blood cells. The problems arise when that emergency response, crucial to repelling pillagers in the short term, drags on indefinitely. Imagine it this way. Your dinner is cooking on the stove. You’re paying bills. You smell smoke. You jump up, leaving those tasks half-done, and search for the fire before it burns down your house. Normally, once you put the fire out, you’d return to your tasks and then eat dinner.
But now imagine that you never find the fire, and you never stop smelling the smoke. You remain in a perpetual state of alarm. Your bills never get paid. You never eat your dinner. Your house smolders. Your life falls into disarray.
That’s metabolic syndrome. Normal function ceases. Aging accelerates. Diabetes develops. Heart attacks strike. The brain degenerates. Life ends early. And it’s all driven, in this understanding, by chronic, low-grade inflammation.
Where does the perceived threat come from—all that inflammation? Some ingested fats are directly inflammatory. And dumping a huge amount of calories into the bloodstream from any source, be it fat or sugar, may overwhelm and inflame cells. But another source of inflammation is hidden in plain sight, the 100 trillion microbes inhabiting your gut. Junk food, it turns out, may not kill us entirely directly, but rather by prompting the collapse of an ancient and mutually beneficial symbiosis, and turning a once cooperative relationship adversarial.
We’re already familiar with a version of this dynamic: cavities. Tooth decay is as old as teeth, but it intensified with increased consumption of refined carbohydrates, like sugar, just before and during the industrial revolution. Before cheap sugar became widely available, plaque microbes probably occupied the warm and inviting ecological niche of your mouth more peaceably. But dump a load of sugar on them, and certain species expand exponentially. Their by-product—acid—which, in normal amounts, protects you from foreign bacteria—now corrodes your teeth. A once cooperative relationship becomes antagonistic.
Something similar may occur with our gut microbes when they’re exposed to the highly refined, sweet, and greasy junk-food diet. They may turn against us.
A DECADE AGO, microbiologists at Washington University in St. Louis noticed that mice raised without any microbes, in plastic bubbles with positive air pressure, could gorge on food without developing metabolic syndrome or growing obese. But when colonized with their native microbes, these mice quickly became insulin resistant and grew fat, all while eating less food than their germ-free counterparts.
The researchers surmised that the microbes helped the rodents harvest energy from food. The mice, which then had more calories than they needed, stored the surplus as fat. But across the Atlantic, Patrice Cani at the Catholic University of Louvain in Brussels, Belgium, suspected that inflammation contributed, and that the inflammation emanated from native microbes.
To prove the principle, he gave mice a low dose of endotoxin, that molecule that resides in the outer walls of certain bacteria. The mice’s livers became insulin resistant; the mice became obese and developed diabetes. A high-fat diet alone produced the same result: Endotoxin leaked into circulation; inflammation took hold; the mice grew fat and diabetic. Then came the bombshell. The mere addition of soluble plant fibers called oligosaccharides, found in things like bananas, garlic, and asparagus, prevented the entire cascade—no endotoxin, no inflammation, and no diabetes.
Oligosaccharides are one form of what’s known as a “prebiotic”: fibers that, because they make it all the way to the colon intact, feed, as it were, the bacteria that live there. One reason we’ve evolved to house microbes at all is because they “digest” these fibers by fermenting them, breaking them down and allowing us to utilize their healthful byproducts, like acetic acid, butyric acid, B vitamins, and vitamin K.
Cani had essentially arrived at the same place as Dandona with his freshly squeezed orange juice. Only his controlled animal experiments allowed a clearer understanding of the mechanisms. Junk food caused nasty microbes to bloom, and friendly bugs to decline. Permeability of the gut also increased, meaning that microbial byproducts—like that endotoxin—could more easily leak into circulation, and spur inflammation. Simply adding prebiotics enjoyed by a select group of microbes—in this case, Bifidobacteria—kept the gut tightly sealed, preventing the entire cascade. The fortified bacteria acted like crowd-control police, keeping the rest of the microbial mob from storming the barrier.
“If we take care of our gut microbiota, it will take care of our health,” Cani says. “I like to finish my talks with one sentence: ‘In gut we trust.'”
So our sweet and greasy diet—almost certainly without evolutionary precedent—doesn’t just kill us directly: It also changes gut permeability and alters the makeup of our microbial organ. Our “friendly” community of microbes becomes unfriendly, even downright pathogenic, leaking noxious byproducts where they don’t belong. H.G. Wells would be proud of this story—the mighty Homo sapiens felled by microscopic life turned toxic by junk food. It’s nothing personal; the bugs that bloom with an energy-dense diet may act in their own self-interest. They want more of that food sweet, fatty food on which they thrive.
AROUND THE TIME when Paresh Dandona began puzzling over the immune response to a fast-food breakfast, a Chinese microbiologist named Liping Zhao was realizing that he needed to change how he ate, or he might drop dead. He was 44 pounds overweight, his blood pressure was elevated, and his “bad” cholesterol was high.
He caught wind of the studies at Washington University in St. Louis suggesting that microbes were central to obesity. The research jibed with ancient precepts in Chinese medicine that viewed the gut as central to health. So Zhao decided on a hybridized approach—some 21st-century microbiology topped with traditional Chinese medicine.
He changed his diet to whole grains, rich in those prebiotic fibers important for beneficial bacteria. And he began regularly consuming two traditional medicinal foods thought to have such properties: bitter melon and Chinese yam.
Zhao’s blood pressure began normalizing and his “bad” cholesterol declined. Over the course of two years, he lost 44 pounds. He sampled his microbes throughout. As his metabolism normalized, quantities of a bacterium called Faecalibacterium prausnitzii increased in his gut. Was its appearance cause or consequence? Others have observed that this bacterium is absent in people suffering from inflammatory diseases, such as Crohn’s disease, as well as Type 2 diabetes. Scientists at the University of Tokyo have shown that colonizing mice with this bacterium and its relatives—called “Clostridium clusters”—protects them against colitis. But still, evidence of causation was lacking.
Then one day in 2008, a morbidly obese man walked into Zhao’s lab in China. The 26-year-old was diabetic, inflamed, had high bad cholesterol, and elevated blood sugar. No one in his immediate family was heavy, but he weighed 385 pounds.
Zhao noticed something odd about the man’s microbes. Thirty-five percent belonged to a single, endotoxin-producing species called Enterobacter cloacae. So he put the man on a version of his own regimen—whole grains supplemented with other prebiotics. As treatment progressed, the Enterobacter cloacae declined, as did circulating endotoxin and markers of inflammation.
After 23 weeks, the man had lost 113 pounds. That bacterial bloom had receded to the point of being undetectable. Counts of anti-inflammatory bacteria—microbes that specialize in fermenting nondigestible fibers—had increased. But could Zhao prove that these microbial changes caused anything? After all, the regimen may have simply contained far fewer calories than the patient’s previous diet.
So Zhao introduced the Enterobacter into mice. They developed endotoxemia, fattened up and became diabetic—but only when eating a high fat diet. Mice colonized with bifidobacteria and fed a high fat diet, meanwhile, remained lean, as did germ-free mice. The enterobacter was evidently unique, an opportunist. Aided by a high fat diet, the microbe appeared able to hijack the metabolism of both mice and man.
Zhao, who related his own story to Science last year, has repeated a version of this regimen in at least 90 subjects, achieved similar improvements, and has more than 1,000 patients in ongoing trials. He declined to be interviewed for this article, saying that the response to his research, both by press and individuals seeking advice, had been overwhelming. “I receive too many emails to ask for help but I can not provide much,” he wrote in an email. “I feel very bad about this and would like to concentrate on my research.”
Other researchers have tried an even more radical approach to treating the microbiome: the fecal transplant. It was originally developed to treat the potentially life-threatening gut infection caused by the bacterium Clostridium difficile. Studies so far suggest that it’s 95 percent effective in ousting C. diff. and has no major side effects. “Fecal engraftment” is now being considered a method for rebooting microbiota generally. Scientists at the Academic Medical Center in Amsterdam mixed stool from lean donors with saline solution and, via a tube that passed through the nose, down the throat and past the stomach, introduced the mixture to the small intestine of nine patients with metabolic syndrome. Control subjects received infusions of their own feces.
Those who received “lean” microbes saw improvements in insulin sensitivity, though they didn’t lose weight and saw the improvements disappear within a year. But Max Nieuwdorp, senior author on the study, aims to conduct the procedure repeatedly to see if the “lean” microbes will stick. And when he’s identified which are important, he hopes to create an anti-obesity “probiotic” to be taken orally.
Probiotics are just bacteria thought to be beneficial, like the lactobacilli and other bacteria in some yogurts. In the future probiotics might be bacteria derived from those found in Amazonian Indians, rural Africans, even the Amish—people, in other words, who retain a microbial diversity that the rest of us may have lost. Already, the literature suggests that a gold rush has begun—a flood of what you might call “fecoprospectors” seeking to catalog and preserve the diversity and richness of the ancestral microbiota before it is lost in the extinction wave sweeping the globe.
Ultimately, the strongest evidence to support microbial involvement in obesity may come from a procedure that, on the face of it, has nothing to do with microbes: gastric bypass surgery. The surgery, which involves creating a detour around the stomach, is the most effective intervention for morbid obesity—far more effective than dieting.
Originally, scientists thought it worked by limiting food consumption. But it’s increasingly obvious that’s not how the procedure works. The surgery somehow changes expression of thousands of genes in organs throughout the body, resetting the entire metabolism. In March, Lee Kaplan, director of the Massachusetts General Hospital Weight Center in Boston, published a study in Science Translational Medicine showing a substantial microbial contribution to that resetting.
He began with three sets obese mice, all on a high-fat diet. The first set received a sham operation—an incision in the intestine that didn’t really change much, but was meant to control for the possibility that trauma alone could cause weight loss. These mice then resumed their high fat diet. A second set also received a sham operation, but was put on a calorically restricted diet. The third group received gastric bypass surgery, but was then allowed to eat as it pleased.
As expected, both the bypass mice and dieted mice lost weight. But only the bypass mice showed normalization of insulin and glucose levels. Without that normalization, says Kaplan, mice and people alike inevitably regain lost weight.
To test the microbial contribution to these outcomes, Kaplan transplanted the microbiota from each set to germ-free mice. Only rodents colonized with microbes from the bypass mice lost weight, while actually eating more than mice colonized with microbes from the other groups.
In humans, some studies show a rebound of anti-inflammatory bacteria after gastric-bypass surgery. Dandona has also noted a decline in circulating endotoxin after the procedure. “I would never argue, and won’t argue, that all the effects of the gastric bypass can be transferred by the microbiota,” says Kaplan. “What we’ve found is the first evidence that any can. And these ‘any’ are pretty impressive.” If we understand the mechanism by which the microbiota shifts, he says, perhaps we can induce the changes without surgery.
NOW, NOT EVERYONE ACCEPTS that inflammation drives metabolic syndrome and obesity. And even among the idea’s proponents, no one claims that all inflammation emanates from the microbiota. Moreover, if you accept that inflammation contributes to obesity, then you’re obligated to consider all the many ways to become inflamed. The odd thing is, many of them are already implicated in obesity.
Particulate pollution from tailpipes and factories, linked to asthma, heart disease, and obesity, is known to be a cause of inflammation. So is chronic stress. And risk factors may interact with each other: In macaque troops, the high-ranking females, which experience less stress, can eat more junk food without developing metabolic syndrome than the more stressed, lower-ranking females. Epidemiologists have made similar observations in humans. Poorer people suffer the consequences of lousy dietary habits more than do those who are wealthier. The scientists who study this phenomenon call it “status syndrome.”
Exercise, meanwhile, is anti-inflammatory, which may explain why a brisk walk can immediately improve insulin sensitivity. Exercise may also fortify healthy brown fat, which burns off calories rather than storing them, like white fat does. This relationship may explain how physical activity really helps us lose weight. Yes, exercise burns calories, but the amount is often trivial. Just compensating for that bagel you ate for breakfast—roughly 290 calories—requires a 20-minute jog. And that’s not counting any cream cheese. Sleep deprivation may have the opposite effect, favoring white fat over brown, and altering the metabolism.
Then there’s the brain. Michael Schwartz, director of the Diabetes and Obesity Center of Excellence at the University of Washington in Seattle, has found that the appetite regulation center of the brain—the hypothalamus—is often inflamed and damaged in obese people. He can reproduce this damage by feeding mice a high-fat diet; chronic consumption of junk food, it seems, injures this region of the brain. Crucially, the brain inflammation precedes weight gain, suggesting that the injury might cause, or at least contribute to, obesity. In other words, by melting down our appetite control centers, junk food may accelerate its own consumption, sending us into a kind of vicious cycle where we consume more of the poison wreaking havoc on our physiology.
Of course there’s a genetic contribution to obesity. But even here, inflammation rears its head. Some studies suggest that gene variants that increase aspects of immune firepower are over-represented among obese individuals. In past environments, these genes probably helped us fight off infections. In the context of today’s diet, however, they may increase the risk of metabolic syndrome.
Whether inflammation drives obesity or just contributes, how much of it emanates from our microbiota, or even whether it causes weight gain, or results from it—these are still somewhat open questions. But it is clear that chronic, low-grade inflammation, wherever it comes from, is unhealthy. And as Dandona discovered all those years ago, food can be either pro- or anti-inflammatory. Which brings us back to the question: What should we eat?
FIFTY YEARS AGO, due to the perceived link with heart disease, nutritionists cautioned against consuming animal fats and recommended hydrogenated vegetable oils, such as margarine, instead. Alas, it turned out that these fats may encourage the formation of arterial plaques, while some fats that were discarded—in fish and olive oil, for example—seem to prevent cardiovascular disease and obesity.
As people unwittingly cut out healthy fats, they compensated by consuming more sugar and other refined carbohydrates. But a high-sugar diet can produce endotoxemia, fatty liver, and metabolic syndrome in animals. So that’s yet another reason to avoid refined, sugary foods.
What about popular weight loss regimes, like the Atkins diet, that emphasize protein? In a 2011 study by scientists at the University of Aberdeen, in Scotland, 17 obese men were given a high-protein, low-carb diet. It prompted a decline of anti-inflammatory microbes, whose fermentation byproducts are critical to colonic health, and produced a microbial profile associated with colon cancer. So although it may prompt rapid weight loss, a high-protein, low-carb diet may predispose people to colon cancer. In the rodent version of this experiment, the addition of a prebiotic starch blunted the carcinogenic effect. Again, it’s not only what’s present in your diet that matters, but also what’s absent.
So, should we sprinkle a packet of fiber on our cheeseburger? Dandona has looked at this possibility and says that though this study has not yet been published, he’s found that packeted fiber does, when eaten with a fast-food meal, soften the food’s inflammatory effects. Fast-food companies could, in theory, pack their buns full of prebiotics, shielding their customers somewhat from metabolic syndrome.
But that’s not really what Dandona or anyone else is advocating. The pill approach—the idea that we can capture a cure in a gel cap—may be part of what got us in trouble to begin with. Natural variety and complexity have their own value, both for our own bodies and for our microbes. This may explain why orange juice, which contains plenty of sugur, doesn’t have inflammatory effects while a calorically equivalent quantity of sugar water does. Flavonoids, other phytochemicals, vitamins, the small amount of fiber it carries, and other things we have yet to quantify may all be protective.
To that end, consider a study by Jens Walter (PDF), a scientist at the University of Nebraska-Lincoln. He supplemented the diet of 28 volunteers with either brown rice, barley, or both. Otherwise, they continued eating their usual fare. After four weeks, those who consumed both grains saw increased counts of anti-inflammatory bacteria, improved insulin sensitivity, and reduced inflammation—more so than subjects who just had one grain. Walter doesn’t think it’s an accident that those who ate both barley and brown rice saw the greatest improvement. The combination likely presented microbes with the largest array of fermentable fibers.
Scientists are also intensely interested in concocting “synbiotics,” a mixture of probiotic bacteria and the prebiotic fibers that feed them. This type of combination may already exist in staple dishes and garnishes, from sauerkraut to kefir, in traditional cuisines the world over. In theory, such unpasteurized, fermented foods that retain their microbial communities are a health-producing triple whammy, containing prebiotic fibers, probiotic bacteria, and healthful fermentation byproducts like vitimins B and K. A smattering of recent studies suggest that embracing such grub could protect against metabolic syndrome. In one monthlong trial on 22 overweight South Koreans, unpasteurized fermented kimchi, which is made from cabbage, improved markers of inflammation and caused very minor decreases in body fat. Fresh, unfermented kimchi also helped, but not as much. In another double-blind, placebo-controlled study on 30 South Koreans, a pill of fermented soybean paste eaten daily for 12 weeks decreased that deadly visceral fat by 5 percent. Triglycerides, a risk factor for heart attacks, also declined. An epidemiological study, meanwhile, found that consumption of rice and kimchi cut the odds of metabolic syndrome. It all hints at a future where sauerkraut, kimchi, sour pickles, and other fermented foods that contain live microbial cultures do double duty as anti-obesity medicine.
So what else to eat? Onions and garlic are especially rich in the prebiotic fiber inulin, which selectively feeds good bacteria within. Potatoes, bananas, and yams carry loads of digestion-resistant starches. Apples and oranges carry a healthy serving of polysaccharides (another form of prebiotic). Nuts and whole grains do as well. Don’t forget your cruciferous vegetables (cabbage, broccoli, and cauliflower) and legumes. There’s no magic vegetable. Yes, some plant products are extra rich in prebiotics—the Jerusalem artichoke, for example—but really, these fibers abound in plants generally, and for a simple reason: Plants store energy in them. That’s why they’re resistant to degradation. They’re designed to last. (For more on what foods to eat, see “Should I Take A Probiotic?“)
The very qualities that improve palatability and lengthen shelf life—high sugar content, fats that resist turning rancid, and a lack of organic complexity—make refined foods toxic to your key microbes. Biologically simple, processed foods may cultivate a toxic microbial community, not unlike the algal blooms that result in oceanic “dead zones.”
In fact, scientists really do observe a dead zone of sorts when they peer into the obese microbiota. Microbes naturally form communities. In obese people, not only are anti-inflammatory microbes relatively scarce, diversity in general is depleted, and community structure degraded. Microbes that, in ecological parlance, we might call weedy species—the rats and cockroaches of your inner world—scurry around unimpeded. What’s the lesson? Junk food may produce a kind of microbial anarchy. Opportunists flourish as the greater structure collapses. Cooperative members get pushed aside. And you, who both contain and depend on the entire ecosystem, pay the price.