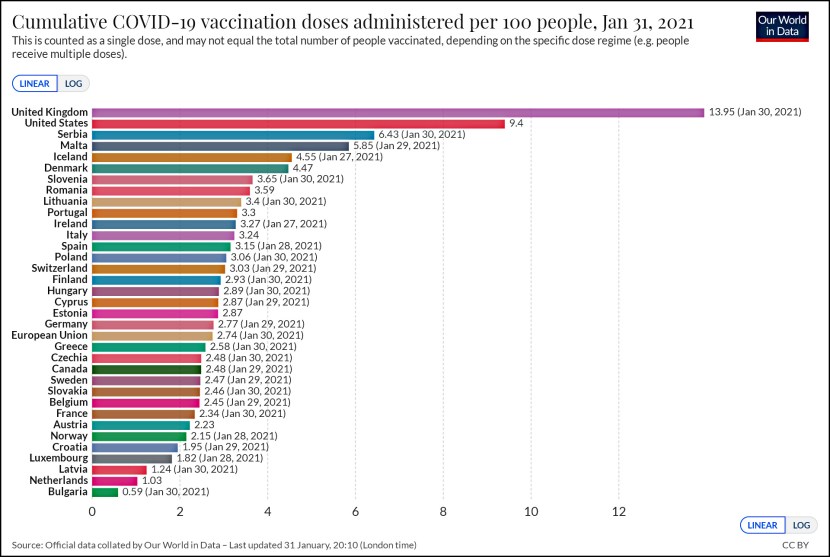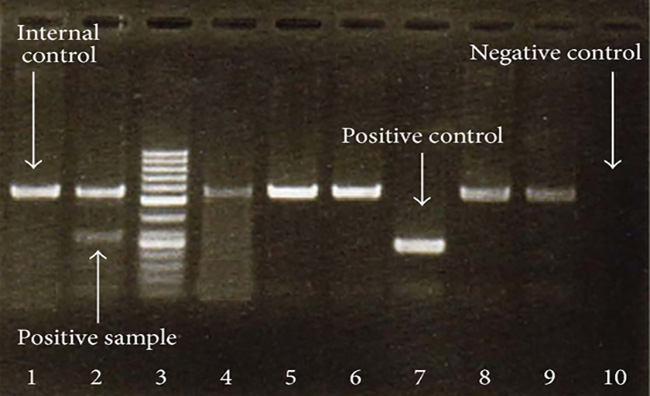
Test results from a PCR assay of a herpes virus.Mohammad Hadi Sadeghian et al., Open access
I finally got curious about how and why the CDC managed to botch COVID-19 testing so badly. The answer isn’t complicated, but I still have questions.
The whole story is pretty short. The CDC test is a PCR¹ assay, a well-known and fairly easy diagnostic to perform. However, to make sure it isn’t simply flagging everything as the target virus, PCR tests always include an unrelated sample of DNA as one of the reagents. If everything is working properly, the unrelated sample should never produce a positive response. Unfortunately, the CDC bit off more than it could chew and created a test for multiple viruses—and then botched the reagents for one of them. The result was lots of false positives, which in turn meant that the CDC restricted use of its test to a very small number of laboratories that followed a very specific protocol. They also declined to allow state labs to simply create their own PCR tests.
Beyond this the details are unimportant except to say that the COVID-19 tests used in other countries are also PCR assays, so it’s not as if the CDC was breaking new ground here. And eventually the CDC agreed to limit its test just to COVID-19, which means it no longer has the false positive problem. This took several weeks, and I’m curious why it took so long. But what I’m really curious about is why the CDC didn’t just approve the use of PCR tests that were already being used in China, Europe, and elsewhere. Is there some reason that the United States of America just has to have its very own test instead of using someone else’s test that’s already in the field?
¹Polymerase Chain Reaction. More precisely, it’s an RT-PCR, or Reverse Transcription PCR, which can be used to detect RNA strands. This is what’s needed in the case of COVID-19.